Going Global: The Challenges of Selling in Multiple Markets, Part 3

Click here for Part I, and here for Part II.
•Front-line duty
•Packaging as brand manifestation
•Necessity breeds creativity
•More languages. More opportunities
•Sell, sell, sell
The medical device industry is going global on a scale unheard of just five years ago. As new markets open, device manufacturers are no longer content to let others "test the waters" before jumping in. Companies now make efforts to launch products globally, with packaging and labeling that work seamlessly across borders. Some of these efforts succeed—with lucrative results; some of these efforts fail, with disastrous cost overruns and launch delays.
For executives who must manage this process, the pressure is only getting worse. For every additional market, there are additional language, regulatory, and logistical issues that must be negotiated. Budgets and schedules are tight; and upper level management often doesn't understand or appreciate the complexity of the issues involved.
But there are success stories out there: device manufacturers that have innovated with new methods of labeling, better translation management processes, and more efficient packaging. And for these firms, companies such as Guidant Vascular Intervention and Sulzer Carbomedics, the rewards of a "globalization strategy" are rich and rewarding.
This is the third in a series of articles examining the challenges and benefits of selling in multiple markets. The first article investigated organizational, business, and resource challenges, while the second article focused on challenges in the areas of international regulatory affairs and translation management.
The trend toward globalization got a kick-start because of—or despite, depending on your point of view—the three medical device directives introduced by the European Union over the past decade. Depending on your products' characteristics, your company may be subject to several overlapping regulations. Although devices are at the center of these directives, the packaging and labeling associated with them play an important role.
Packaging operations are at the front line of their companies' multilingual compliance efforts. To wit:
- Because of shorter product life cycles and faster time-to-market requirements, development of new product is constantly accelerating. Packaging is tasked with squeezing additional efficiencies from suppliers and already-lean production lines.
- Mergers and acquisitions are wreaking havoc with firms' packaging groups. These business combinations redirect resources from operations and, following the combination, at least one of the merging companies they will need to overhaul systems, labeling, and validation procedures.
- While changing regulations affect all departments within medical device companies, the design of safe, effective packaging is at the eye of the regulatory storm. Every time a standard is revised or a new guideline is issued, engineers must review existing packaging for compliance.
- Realizing that over-packaging is a problem for many device manufacturers, corporate cost-savings programs often target the department. Significant savings can be achieved by reducing packaging materials, strategic sourcing, and creating a lean manufacturing strategy.
Packaging as brand manifestation
Fundamentally, medical device packaging has three main goals:
- Maintain the sterile integrity and/or protect the product
- Communicate critical end-user labeling information to the customer
- Promote the brand and company identity
Achieving these goals is difficult enough when working in one language, serving one market. The challenges multiply when developing packaging for global markets, satisfying country-specific language requirements, and reacting to ever-changing corporate marketing priorities.
"It's a moving target—the rules change constantly," states Julieann Thornton, supervisor of package labeling at Guidant Vascular Intervention (Temecula, CA). In part, that is because packaging is a key ingredient to a company's global branding effort. The package is the hands-on manifestation of the brand. As device manufacturers strive to strengthen their global brands and improve gross profit margins, they are apt to try to simplify and streamline global packaging.
Some of the issues surrounding global packaging include:
Products and materials
This kind of "SKU rationalization" is important because the expense of readying products and packaging for global markets is significant:
- The selected packaging materials must adhere to regulatory standards and meet enhanced shelf-life requirements due to longer distribution times.
- A fundamental decision has to be made regarding the use of regional packaging. In short, will one package be used globally or will the firm develop market-specific variants with a customized look and feel (e.g., color, size, design, and copy) for each market or region. This decision has far-reaching financial and marketing implications and must be weighed carefully.
- Packaging changes can have dramatic implications—positive or negative. For instance, even a small reduction in packaging weight (while maintaining product protection) will improve a company's bottom line.
Resource and environmental concerns
Different countries and trading blocks are apt to have often differing and sometimes conflicting views on the importance of packaging waste. The EU Directive on Packaging and Packaging Waste 94/62/EC emphasizes prevention, recovery, and reuse of packaging materials. However, implementation of the Directive has been slow and inconsistent among member countries.
"Some countries impose very restrictive requirements while other countries have barely begun implementing and enforcing the Directive," says Brett White, senior packaging engineer at Sulzer Carbomedics (Austin, TX). According to White, the same medical packaging waste "may be perceived as benign by certain countries while other countries see it as ‘bad' for the environment, thus limiting your choices for material and sterilization method." A good example of this is the EtO (ethylene oxide) method of sterilization, which has raised concerns about its environmental impact.
Operational competence
When developing products for global markets it is critical to clearly define packaging parameters. Special product requirements, differing shelf-life requirements, and international testing and validation constraints impact the development timeline and costs for packaging.
Those device manufacturers that have strong capabilities in this area will have an important advantage over their competitors. Their lower cost of goods sold—typically achieved through automation, source reduction, and process reengineering—provide these firms with the opportunity to aggressively price products and to undersell their competitors.
Supply-chain management
Selling to global markets places new strains on a company's supply chain: sourcing of raw materials, product shipment and transportation, supplier management, and development of an efficient distribution channel all become much more complicated as the number of target markets served increases.
Medical device manufacturers find that going overseas also provides an opportunity to review existing supplier arrangements and begin a supplier reduction initiative. Not all domestic suppliers will be able to support a company's overseas locations. Also, increased sales volumes from overseas sales will allow manufacturers to negotiate volume discounts.
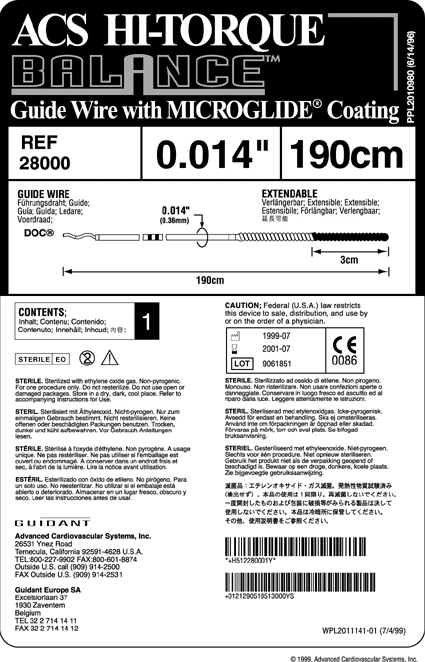
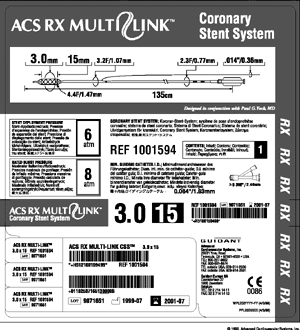
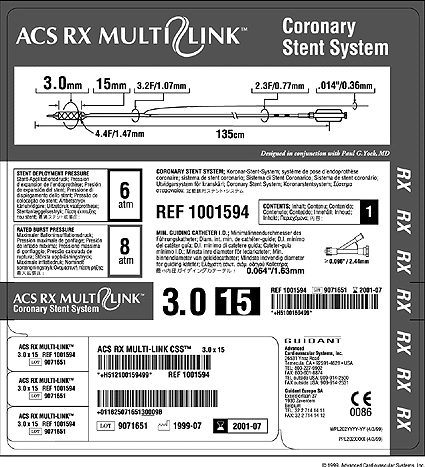
Multilingual labeling
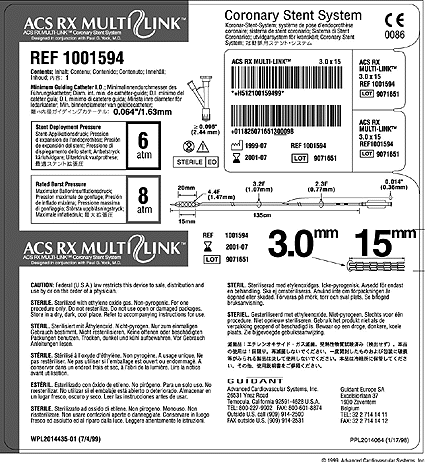
Packaging engineers have spent years trying to minimize the size of device packaging. Now, requirements for squeezing bar codes, icons, and an ever-increasing number of languages on product labels are requiring companies to rethink their multilingual labeling strategy from the ground up.
But this trend is not limited to international labeling alone. There is an increasing polarization international medical device regulations between those that require FDA approval as a precondition for selling device in their market and those that require EU approval. So, because countries like Mexico, Canada, and Taiwan require FDA approval for medical devices being sold there, some manufacturers may produce one label for all "FDA markets". This would mean that products sold in the U.S. would feature Chinese, French, Spanish and potentially other languages.
Label real estate has gotten so scarce that Julieann Thornton of Guidant Vascular Intervention proclaims that many products have "absolutely no more labeling real estate left."
For medical device companies, this is of serious concern. As labels get denser, their clarity suffers, making necessary information harder to find for the customer. Unwilling to increase the size of label stock used (and with it, packaging costs), this shortage of label real estate is convincing companies to adapt new labeling strategies.
Guidant Vascular Intervention, for instance, has begun to preprint some "boilerplate" text on boxes and pouches. This approach frees up much needed space on the label. Other approaches under consideration include:
- Single-language or regional labeling, i.e., grouping languages by geographic area, depending on the supporting distribution network.
- "Pack-to-demand," meaning that a generic label is affixed at the manufacturing facility and that additional more specific labeling and documentation are added at the shipment/distribution point.
- Labeling at the distributor level may or may not be a feasible alternative for certain overseas offices. While there are strong incentives for this approach—handing off part or all translation management, cleaner cost accounting, etc.—there are also significant drawbacks. Affiliate offices may lack the infrastructure, resources, or skills to take on this additional responsibility.
Medical device manufacturers are anxious about reversing the path toward global packaging. The prospect of increased inventory expense and materials waste and lower production efficiencies is worrisome. Nonetheless, the writing is on the wall and many companies are likely to adapt some of these or similar approaches.
More languages. More opportunities.
Multiple language requirements are at the heart of the labeling challenge. And it's no wonder that labels are getting cramped: Sulzer Carbomedics labels, for example, feature up to 14 languages.
The cost associated with these multiple languages is substantial. Some of the expense items include:
- Cost of translation—this includes direct costs (to translation companies, printer vendors) and indirect expenses (for company internal staffing, infrastructure, etc.);
- Technology—especially the hardware and software needed to integrate "double-byte" Asian and Middle-Eastern languages in the production line;
- Label stock—all things being equal, multilanguage labels are larger than single-language labels, requiring more expensive labels and larger, more expensive packaging.
The introduction of additional languages comes as a direct result of international regulatory requirements. As these requirements are constantly changing, device manufacturers devote significant resources to remain compliant. According to White at Sulzer Carbomedics, "it is difficult to maintain compliance with international regulations governing packaging, labeling and supplemental documentation that must accompany the device (e.g., Instructions for Use)."
But it is no longer simply direct regulatory requirements that cause device companies to add languages. Increasingly, device manufacturers respond to what Guidant's Thornton refers to as "derived requirements." Even though a particular country may not stipulate the translation of labeling information, end-customers in the country require it. Manufacturers are seeing this as an opportunity to gain a leg up on competitors—or risk losing ground.
Today, "time to market" refers to the world, not just the United States. As packaging becomes more and more challenging, device manufacturers must continue to innovate to offset additional expenses. Marketing will also play a more significant role, particularly in countries where brand and company awareness are limited. The Internet will play a major role in product and sales support. And the companies who embrace these new forms of communication, in all languages, will be embraced by the markets they serve.
The final article in this series examines challenges in the areas of sales, marketing, and after-sale support and will investigate some of the specific challenges brought about by the merger craze sweeping the medical-device industry.
